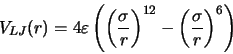
The Lennard-Jones pair potential is given by
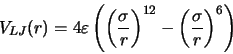
Lennard-Jones type interactions are assumed for Ar-Ar and Ar-Cu. The
parameters are for Ar
![]() Å,
Å,
![]() K [1] (Maitland, 1981)
and for Cu
K [1] (Maitland, 1981)
and for Cu
![]() Å,
Å,
![]() K (cf. homework #2).
From the mixing rules of Lorentz and Berthelot [1] one
approximates the values for Ar-Cu:
K (cf. homework #2).
From the mixing rules of Lorentz and Berthelot [1] one
approximates the values for Ar-Cu:
![]() Å,
Å,
![]() K.
K.
The potentials were evaluated numerically. In the code the energies and forces were calculated using spline interpolation.